Export of GlossWell Type Anti-Viral from Japan
Please let more people know about this page!
GlossWell #360 / #240 / #750 Type Anti-Viral is a paint. Since paint is classified as a hazardous material internationally, the cost of exporting it from Japan by air or sea is very high, and it is very difficult for an individual to handle the payment of freight and customs clearance for importation.
In this page, we are announcing a special paint that contains a very effective anti-bacterial compound against COVID-19 : GlossWell #360 / #240 / #750 Type Anti-Viral to corporations, trading companies and government officials in your country. We are disclosing all the necessary evidence to prove the efficacy against COVID-19. All of this evidence has been tested and obtained by public institutions in Japan.
GlossWell #360 / #240 / #750 Type Anti-Viral forms a very tough and special coating with anti-bacterial properties. The coating will adhere to the surface for a long time. If a similar special coating does not exist in your country, please inform any person or entity that can import GlossWell #360 / #240 / #750 Type Anti-Viral or any government official of the existence of this page!
List of various test data
Various tests on the anti-virus and anti-bacterial performance of this coating were conducted at the Kobe Testing Center / Microorganism Testing Laboratory of the Japan Textile Products Quality Technology Center. Details of the test results are shown below.
Microorganisms to be tested
- Antiviral test: COVID-19
- Antiviral test: influenza A virus (with envelope)
- Antiviral test : Feline calicivirus (without envelope membrane)
- Antibacterial test : Pseudomonas aeruginosa
- Antibacterial test : E. coli (O157:H7)
- Antifungal test : Black mold
Antiviral test : COVID-19
◯ Sample submission: November 4, 2020 / Response date: December 28, 2020
◯ Client: Presence, Inc.
◯ Test item: Antiviral activity test
◯ Test method: ISO21702 / Measurement of antiviral activity on plastics and other non-porous surfaces
◯ Test organization: QTEC, Japan Textile Products Quality Technology Center, Kobe Testing Center, Microorganism Testing Laboratory
【 Test Summary 】
◯ Test virus: Severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) / NIID isolate:
JPN/TY!WK-521 (distributed by the National Institute of Infectious Diseases)
・Host cell:VeroE6/TMPRSS2 JCRB1819
・Cytoplasmic drop : Dulbecco’s modified Eagle’s medium (low-glucose) ; DMEM
(SIGMA, Cat#D6046) Minimum Essential Medium Eagle ; EMEM (SIGMA, Cat#-M4655)
・Fetal Bovine Serum : Fetal Bovine Serum (FBS) (SIGMA, Cat#l 73012)
・Sealing film: Polyethylene film
・Control sample : GlossWell #360 Type Anti-Viral (Unprocessed products)
・Test sample : GlossWell #360 Type Anti-Viral (Processed products)
・Purification of test specimen: Not performed
・Inoculation of test virus suspension: 0.4ml
・Test condition: Action temperature: 25°C
・Test condition: Action time: 24 hours (The virus infection titer was also measured immediately after inoculation for the control sample.
・Washing solution: SCDLP diluted 10 times in DMEM containing 2% FBS
・Infection titer measurement method: Plaque measurement method
【 Test Operation 】
1 ) Main Test :
1. Infect host cells with the virus, add EMEM and incubate at 37°C for a predetermined period of time, then centrifuge at 4°C, l,000xg for 15 minutes, and the supernatant is used as the test virus suspension.
2. Dilute the virus suspension obtained in 1. 10-fold with sterile distilled water to 1 ~sx101 PFU/mL, and use this as the test virus suspension.
3. Place each specimen (50 mm x 50 mm) on the bottom of a sterile Petri dish with the processed side up, and inoculate with 0.4 ml of the test virus suspension.
4. Cover the petri dish with an adhesive film (40 mm x 40 mm) and press down lightly so that the test virus suspension is spread over the entire film.
5. Cover the petri dish with the lid.
6. After dying at 25°C for 24 hours at 90%RH or higher, add 10 mL of washing-up liquid to each test sample.
7. Scrub the surface of each test specimen and the dense cling film to wash out the virus.
8. Dilute the washout solution 10-fold using DMEM containing 2% FBS.
9. Determine the viral infection titer by the plaque measurement method.
2 ) Host cell verification test :
2 )-1 Cytotoxicity Confirmation
1. Add l0mL of washing-up liquid to each test sample, and perform the washing-up procedure as in the main test.
2. Make a 10-fold dilution of the washout solution using DMEM containing 2% FBS.
3. Stain the cells as in the plaque measurement method to check for cytotoxicity.
2 )-2 Susceptibility testing of cells to viruses
1. Add 10mL of washing-up liquid to each test sample and perform the washing-up procedure as in the main test.
2. Dilute the washout solution 10-fold using DMEM containing 2% FBS.
3. Take 5 mL of the solution in 2. above into a sterile test tube.
4. Prepare a test virus suspension at 4~6×104 PFU/ml using EMEM, and add 0.05 ml of the suspension to the washout solution in 2.
5. Allow to stand at 25°C for 30 minutes.
6. Measure the virus infection titer by the plaque measurement method and confirm the susceptibility of the cells to the virus by measuring the virus infection titer per ml of washout solution.
【 Test Results 】
1 ) Main Test :
・Test virus: SARS-CoV-2 NIID isolate; JPN/TY!WK-521 (distributed by the National Institute of Infectious Diseases)
・Test virus suspension concentration: 1.2 x 107 PFU/ml
| Specimen | Virus infection titer (PFU/cm2) (Note 2) Logarithmic value for normal use | Virus infection titer (PFU/cm2) (Note 2) log-average normalized value | Antiviral activity value 【R】 (Note 2) |
|---|---|---|---|
| GlossWell #360 Type Anti-Viral (Unprocessed) (Note 1) Immediately after inoculation【U0】 | n1 / 5.52 | 5.53 | |
| same as above | n2 / 5.52 | 5.53 | |
| same as above | n3 / 5.55 | 5.53 | |
| GlossWell #360 Type Anti-Viral (Unprocessed product) (Note 1) After leaving for 24 hours【Ut】 | n1 / 5.04 | 5.04 | |
| same as above | n2 / 5.03 | 5.04 | |
| same as above | n3 / 5.06 | 5.04 | |
| GlossWell #360 Type Anti-Viral (Processed product) After leaving for 24 hours【At】 | n1 <1.80 | <1.80 | |
| same as above | n2 <1.80 | <1.80 | |
| same as above | n3 <1.80 | <1.80 | ≧3.2 [ Numeric Description ] |
The antiviral activity value ≧3.2 means that the antiviral activity value after 24 hours is 99.9% or more than 1/1000.
※ The antiviral activity value that is considered acceptable by ISO 21072 is ≧2.0, so the results of this test far exceed the acceptable value.
(Note 1) : GlossWell #360 Type Anti-Viral (unprocessed) (provided by the client) was used as a control sample.
(Note 2) PFU : plaque forming units.
(Note 3) Antiviral activity value R= Ut -At
2 ) Host cell verification test
・Test virus: SARS-CoV-2 NIID isolate; JPN/TY/WK-521 (distributed by the National Institute of Infectious Diseases)
・Test virus suspension concentration: 4.9 x 104PFU/ml
| Specimen | 2 ) -1 Presence of cytotoxicity | 2 ) -2 Confirmation of cell susceptibility to the virus. Virus infection titer (PFU/ml) (Note 2) Logarithmic mean value for normal use | Determination of test validity |
|---|---|---|---|
| GlossWell #360 Type Anti-Viral (Unprocessed) (Note 1) | non- | 【 Su 】 2.68 | establishment |
| GlossWell #360 Type Anti-Viral (Processed product) | non- | 【 Su 】 2.69 | establishment |
| Negative control (Note 4) | non- | 【 Sn 】 2.67 |
(Note 4) A solution of SCDLPs diluted 10-fold in DMEM containing 2% FBS was used as a negative control.
[ Conditions for completion of the test ]
2-1 ) Cytotoxicity: None
2-2 ) Confirmation of cell susceptibility to the virus: | Sn – Su | ≤ 0.5 and | Sn – S1 | ≤ 0.5
【 Reference Information 】
◯ Real-time RT-PCR measurement of virus suspensions subjected to this test.
・Test virus: SARS-CoV-2NIID isolate; JPN/TY/WK.-521 (distributed by the National Institute of Infectious Diseases)
・Virus suspension wastiness: >lOSPFU/ml
・Real-time PCR equipment: Thermal Cycler Dice Real Time SysteM 3 (TaKaRa)
・Detection Kit: SARS-CoV-2Detection Kit -Nl set- (Code NCV-301; Lot# 038200)
(TOYOBO CO.,LTD. Biotech support Department)
◯ Measurement results
The amplification of viral RNA was confirmed by the real-time RT-PCR measurement results (Fig. l).
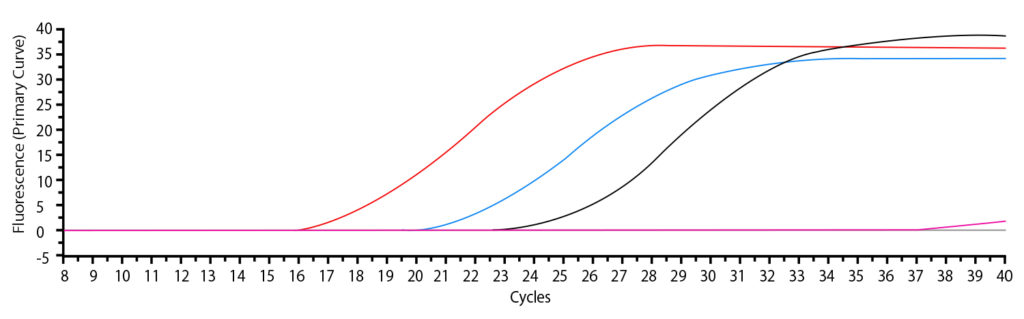
◯ Graph: red line (102-fold dilution of viral suspension flooding in PBS)
◯ Graph: Blue line (103-fold dilution of virus suspension wastage in PBS)
◯ Graph: black line (104-fold dilution of virus suspension wastage in PBS)
◯ Graph: pink line (Negative control; EMEM)
Testing organization: Microbiological Testing Laboratory, Kobe Testing Center, Japan Textile Quality Technology Center
Antiviral test: Influenza A virus (with an envelope membrane)
◯ Specimen: April 2, 2020 / Reply date: June 5, 2020
◯ Test item: Antiviral test
◯ Test details: Evaluate the antiviral properties of polycarbonate sheets
◯ Test method: ISO21702 / Measurement of antiviral activity on plastics and other non-porous surfaces
◯ Testing organization: Microbiology Laboratory, Kobe Testing Center, Japan Textile Quality Technology Center
[ Test Summary ]
・Study virus: Influenza A virus (H3N2) A/Hong Kong/8/68; TC adapted ATCC VR-1679
・Host cells : MDCK cells (canine kidney-derived cells)
・Test sample
(1) GlossWell #360 Type Anti-Viral / Polycarbonate sheet (unprocessed) / control: Sample submitted by the client
(2) GlossWell #360 Type Anti-Viral / Polycarbonate Board (Processed)
・SCDLP medium
・Leaving conditions : Leaving temperature 25°C
・Time of storage 24 hours : (1) GlossWell #360 Type Anti-Viral polycarbonate sheet (unprocessed) was measured immediately after leaving the product for 24 hours.
・Sample size : 5cm x 5cm
・Adhesion film: Polyethylene (4cm x 4cm)
・Inoculation volume of the test virus suspension: 0.4 mL
・Cleanliness of the test specimens: Not conducted.
[ Test Operation: Main Test ]
1. Infect host cells with the virus and culture them, and remove the remaining cells by centrifugation to make a virus suspension.
2. dilute the virus suspension 10-fold with sterile distilled water to a concentration of 1-5X10∧7 PFU/mL, and treat as a test virus suspension.
3. Place each specimen on the bottom of a sterile petri dish with the processed side up, and inoculate it with 0.4 mL of the test virus suspension.
4. Cover the Petri dish with adhesive film and press down lightly so that the test virus suspension is spread over the entire film.
5. Cover the petri dish with the lid.
6. Add 10 mL of washout solution to each test specimen after 24 hours at 25°C.
7. Scrape the surface of each test specimen and the contact film to remove the virus.
8. Measure the viral titer by the plaque assay.
[ Host cell verification test ]
2 )-1 Cytotoxicity confirmation test
1 ) Add 10mL of washout solution to each test specimen, and perform the washout procedure as in this test. 2) Stain cells as in the plaque assay, and confirm the presence of cytotoxicity.
2 ) Stain cells in the same way as the plaque assay and confirm the presence or absence of cytotoxicity.
2 )-2 Confirmation of cell susceptibility to viruses
1. Add 10mL of washing-up liquid to each test specimen, and perform washing-up operation as in this test.
2. 5 mL of the above washout solution is placed in a sterile test tube.
3. Prepare 4-6 X 10 ∧ 4 PFU/mL test virus suspension, and add 0.05 mL of the suspension to the washout solution (2).
4. let it stand at 25°C for 30 minutes.
5. confirm the susceptibility of the cells to the virus by measuring the viral infection titer by the plaque assay.
[ Test method ]
1 ) Main test
・Test virus: Influenza A virus (H3N2), A/Hong Kong/8/68; TC adapted ATCC VR-1679
・The concentration of the test virus suspension: 3.5×10∧7 PFU/ml
| Specimen | Viral infection titer (PFU/cm2) (Note 2) logarithmic mean | Test results : Antiviral activity value [R] (Note 3) | |
| 1) GlossWell #360 Type Anti-Viral / (2) Polycarbonate sheets (unprocessed) (Note 1) | Immediately after inoculation [ Uo ] | 5.77 | ー |
| (1) GlossWell #360 Type Anti-Viral / (2) Polycarbonate sheets (unprocessed) (Note 1) | After leaving it for 24 hours [ Ut ] | 5.41 | ー |
| 2) GlossWell #360 Type Anti-Viral / Polycarbonate sheet (fabricated product) | After leaving it for 24 hours [ At ] | < 0.80 | ≥4.6 [ Explanation of the values ] |
( Note 1 ) GlossWell #360 Type Anti-Viral / polycarbonate plate (unprocessed) as a control sample
– control : sample submitted by the client).
( Note 2 ) PFU : plaque forming units
( Note 3 ) Antiviral activity value R = Ut – At
2 ) Host cell verification test
・Test virus: Influenza A virus (H3N2), A/Hong Kong/8/68; TC adapted ATCC VR-1679
・The concentration of the test virus suspension: 4.0×10∧4 PFU/mL
| Specimen | 2)-1 Cytotoxicity | 2)-2 Confirmation of cell susceptibility to viruses | Determination of test success |
| Virus infection titer (PFU/mL) (Note 2) Logarithmic mean of normal use | |||
| (1) GlossWell #360 Type Anti-Viral / Polycarbonate sheet (unprocessed) | non- | non- | establishment |
| (2) GlossWell #360 Type Anti-Viral / (3) Polycarbonate sheet (fabricated product) | non- | [ St ] 2.48 | establishment |
| Negative control (Note 4) | non- | [ Sn ] 2.60 |
( Note 4 ) SCDLP medium was used as a negative control.
[ Conditions for acceptance of the study ]
2-1) Cytotoxicity : None
2-2) Confirmation of cell susceptibility to virus : | Sn – Su | ≤ 0.5 and | Sn – St | ≤ 0.5
Testing organization: Microbiological Testing Laboratory, Kobe Testing Center, Japan Textile Quality Technology Center
Antiviral test: feline calicivirus (without envelope)
[ Exam summary ]
・ Test virus: Feline calicvirus; Strain: F-9 ATCC VR-782
・ Host cells: CRFK cells (cat kidney-derived cells)
・ Test sample: ① Paint GlossWell # 360 Type Anti-Viral / ② Glass plate
・ Washing solution: SCDLP medium supplemented with Fetal Bovine Serum to a final concentration of 10%
・ Adhesive film: polyethylene (4cm × 4cm)
Norovirus is rare for cell culture, so it is not possible to test, measure and evaluate the effect of various disinfectants or individual concentrations. For that reason, caliciviruses and mouse norovirus, which are closely related, are our testing, measuring and evaluating the disinfecting effect of general disinfectants.
[ Test method ]
1 ) Main test
1. Prepare the test virus suspension.
2. Place the sterilizing agent filter paper on the bottom of the sterile petri dish, add 4.5 mL of sterile ion-exchanged water, place a U-shaped glass tube so that the test piece does not touch the filter paper for humidity control, and place a processing surface on it. Place the test sample on top.
3. Inoculate 0.4 mL of the test virus suspension into each sample.
4. Cover with the adhesive film and press gently so that the test virus suspension spreads over the entire film.
5. Cover the Petri dish.
6. After leaving at ℃ 25 ℃ for 24 hours put the specimen into the sterilizer stomacher bag, and wash out the virus from the specimen by adding 10mL of washing solution.
7. Measure the virus infectivity by the plaque assay.
2 ) Host cell verification test:
2 ) -1 Cytotoxicity confirmation test
1. Put the sample in a sterilizer stomacher bag, add 10 mL of the washing solution, and perform the washing operation in the same manner as in this test.
2. Incubate at room temperature for 30 minutes.
2. Stain the cells in the same manner as in the plaque assay and check for cytotoxicity.
2 ) -2 Confirmation test of cell susceptibility to -2 virus
1. Put the sample in a sterilizer stomacher bag, add 10 mL of the washing solution, and perform the washing operation in the same manner as in this test.
2. Transfer 5 mL of the above washing solution to a sterilized test tube.
3. Prepare the test virus suspension at 5 x 104 PFU / mL, and add 0.05 mL of the suspension to the washing solution in step 2.
4. Leave at room temperature for 30 minutes.
5. Measure the virus infectivity by the -plaque assay to confirm the sensitivity of the cells to the virus.
[ Test result ]
1 ) Main test
Test virus suspension: Feline calicvirus 1.0 x 107 PFU / mL
| Specimen | Virus infection titer (PFU / mL) (Note 2) Common logarithmic mean | |
| Glass plate (Note 1) | Immediately after inoculation | 6.47 |
| Glass plate (Note 1) | After leaving for 24 hours | 4.11 |
| GlossWell # 360 Type Anti-Viral painted piece | After leaving for 24 hours | >2.00 [ Explanation of the values ] |
2 ) Host cell verification test:
| Sample | 2)-1 Cytotoxicity | 2)-2 Confirmation of cell susceptibility to virus |
| Sample | 2)-1 Cytotoxicity | Viral infection titer (PFU/mL) (Note 2) Logarithmic mean of normal use |
| Glass plate (Note 1) | None | 2.44 |
| GlossWell # 360 Type Anti-Viral Paint piece | None | 2.41 |
( Note 1 ) ① A glass plate was used as a control sample.
( Note 2 ) PFU: plaque forming units
2 ) -1
From the results of the cytotoxicity confirmation test, no cytotoxicity was confirmed in any of the samples. In addition, from the results of the test for confirming the sensitivity of the cells to 2) -2 virus, no remarkable decrease in the sensitivity of the cells to the virus was observed in any of the samples.
Testing organization: Microbiological Testing Laboratory, Kobe Testing Center, Japan Textile Quality Technology Center
Antibacterial test / Pseudomonas aeruginosa
[ Test method ]
* Antibacterial test JIS Z 2801 (Film adhesion method)
・ Test strain: Pseudomonas aeruginosa NBRC3080
・ Bacterial solution adjustment solution: 1 / 500NB medium
・ Test bacterial solution inoculation volume: 0.4ml
・ Unprocessed sample: polyethylene film
[ Test results ]
| Test sample | Number of visible bacteria Log average | Antibacterial activity value [R] (Note 2) | |
| Unprocessed specimen (Note 1) | Immediately after inoculation | [U0] 3.87 | - |
| Unprocessed specimen (Note 1) | After culturing for 24 hours | [Ut] 5.56 | |
| GlossWell # 360 Type Anti-Viral painted piece | After culturing for 24 hours | [At] -0.20 | ≧5.8 [ Explanation of the values ] |
Antimicrobial activity value ≧5.8: Indicates an antimicrobial activity value of 99.999% or 1/100000 or higher
( Note 1 ) A polyethylene film was used as a non-processed test piece.
( Note 2 ) Antibacterial activity value R = Ut-At
Testing organization: Microbiological Testing Laboratory, Kobe Testing Center, Japan Textile Quality Technology Center
Antibacterial test / Escherichia coli (O157: H7)
[ Test method ]
* Antibacterial test JIS Z 2801 (Film adhesion method)
-Test strain: Escherichia coli (serotype II O157: H7, verotoxin type I and type II producing strains)
・ Escherichia coil RIMD 0509952
・ Bacterial solution adjustment solution: 1 / 500NB medium
・ Test bacterial solution inoculation volume: 0.4ml
・ Unprocessed sample: polyethylene film
[ Test results ]
| Test sample | Number of viable bacteria Log average | Antibacterial activity value【R 】 (Note 2) | |
| Unprocessed specimen (Note 1) | Immediately after vaccination | [U0] 3.89 | ー |
| Unprocessed specimen (Note 1) | After culturing for 24 hours | [Ut] 4.77 | |
| GlossWell # 360 Type Anti-Viral painted piece | After culturing for 24 hours | [At] <-0.20 | ≧5.0 [ Explanation of the values ] |
Antimicrobial activity value ≧5.0: Indicates an antimicrobial activity value of 99.999% or 1/100000 or higher.
( Note 1 ) ① A polyethylene film was used as a non-processed test piece.
( Note 2 ) Antibacterial activity value R = Ut-At
Testing organization: Microbiological Testing Laboratory, Kobe Testing Center, Japan Textile Quality Technology Center
Antifungal test / Black mold
[ Test method ]
* Antibacterial test JIS Z 2801 (Film adhesion method)
・ Test strain: Cladosporium cladosporioides NBRC6348 (Black mold)
・ Measurement method: Luminescence measurement method
・ Spore suspension preparation solution: 1/20 SDB medium
・ Spore suspension inoculation volume: 0.4ml
・ Mold spore concentration: 1.0 × 105spores / ml
・ Culture conditions: 25 ° C, 95% RH, 42 hours
・ Unprocessed sample: polyethylene film
[ Test results ]
| Test sample | ATP amount Common logarithmic mean | Growth value 【F 】(Note 2) | ||
| Unprocessed test piece | Immediately after inoculation | [Fa] -11.95 | 2.4 | Antifungal activity 【FS】(Note 1) |
| Unprocessed test piece | After 42 hours of culture | [Fb] -9.58 | ||
| GlossWell # 360 Type Anti-Viral painted piece | Immediately after inoculation | [Fo] -13.59 | ー | ≧2.7 [ Explanation of the values ] |
| GlossWell # 360 Type Anti-Viral painted piece | After culturing for 42 hours | [Fc] -13.91 |
Anti-mold activity value ≧2.7: Anti-mold Indicates that the activity value is greater than 99% or 1/100.
( Note 1 ) Antifungal activity value [FS] = (Fb-Fa) – (Fc-Fo)
( * 2 ) Growth value [F] = Fb-Fa
Testing organization: Microbiological Testing Laboratory, Kobe Testing Center, Japan Textile Quality Technology Center
Film performance
| Test item | Test condition | Test result |
| Hardness | Using Mitsubishi Pencil Uni | 2H |
| Adhesion test | 2 100 squares created, cellophane peel test | 100/100 |
| Shock resistance test | Same as JIS K 5600-5-3 drop test. 300g x 500㎜ (25.4㎜ in diameter) | No abnormalities |
| Acid resistance test | % sulfuric acid aqueous solution spot test, 23 ° C x 6 hours | No abnormalities |
| Solvent resistance | Rubbing test (500g load / 10 reciprocations) | No abnormalities |
| 1) No abnormalities in ethanol | No abnormality | |
| 2) Toluene is normal | No abnormality | |
| 3) Methyl ethyl ketone | No abnormality | |
| Warm water resistant | 40 ° C warm water immersion, 100 hours | No abnormality |
| Stain resistance | Oily magic (black, red) Dry cloth wipe | No abnormality |
| Contaminated with carbon black, color difference between contaminated and non-contaminated surfaces | ⊿E=0.5 less | |
| Weather resistance | Sunshine weatherometer (2000 hours) | 80% gloss retention |
| Cooling / heat cycle | 60 ℃ × 3hr ⇔ -20 ℃ × 3hr (10 cycles) | No abnormality |
| Salt spray resistance | 35 ° C, 5% saline, 500 hours | No abnormality |
| Volume resistivity | Conforms to JIS K6249, Ω ・ cm | 4.0×10 : 15th power |
| Dielectric strength | Dielectric strength KV/0.1mm | 5.8 |
| Antibacterial and antiviral properties | See attached test results | |
| RoHS directive substances | Not contained |
Material: Bond steel plate / Film thickness: 6-8μm / Curing condition: After drying at 80 ℃ for 30 minutes, leave at room temperature for 5 days
* The above values are reference values and not standard values.
Precautions for painting
○ Coating environment: Avoid using in an environment with poor ventilation.
○ Pretreatment: Oil, water, and dirt on the surface of the material should be sufficiently removed by solvent degreasing.
○ Painting: Paint immediately. Leaving it for a long time may cause clogging and uneven coating.
・ Manage the film thickness within the specified range.
○ Drying: Organic gas is generated during drying, so provide sufficient ventilation / exhaust.
○ Storage: Store the paint in a cool and dark place.
・ This paint has the property of reacting with moisture in the air. Please seal it after use.
○ Disposal: Follow the MSDS (Material Safety Data Sheet) for disposal of paint residue and waste liquid.
○ Handling Precautions: Do not use in a place with a fire because it uses a flammable organic solvent.
・ Be careful not to come in contact with skin and mucous membranes, especially eyes, as they are irritating.
・ In case of contact, wash with plenty of water.
○ Others: Refer to the product MSDS for details.
Antiviral and antibacterial test results & Documents
SDS Down Load
Recruiting Distributors & Dealers
Our company offers numerous special paints made using cutting edge Japanese chemical technology. Each one of our paints excels at repelling water and oil and withstands submersion. Furthermore, they are also designed to be ultra bright, hard, UV resistant, and heat resistant as well as aesthetically superior. We can offer high added value along with unprecedented new ideas for final surface finishing on all kinds of base materials. We are currently recruiting overseas distributors to carry these superior Japan-made special paints. If you are interested, please contact us through our Contact Form.
Paint in general is treated as hazardous goods, so for shipping charges, we must make individual estimates by airline according to country and region.
First, please send us the Contact Form below. We will calculate the shipping charges to your nearest airport / Term : CIF.
We will then send you a bill including those shipping charges via paypal. After checking that amount, please decide whether or not to purchase our products.
paypal is our only payment method. You can cancel the order at any time.
Furthermore, please note that it will be about 10~14 days before items are shipped.
Contact : Recruiting Distributors & Dealers
BL HY-COATER
PRESENCE Co., Ltd. / BADLAND
Post : 230-0073
2-39-45 Shishigaya Tsurumi-ku Yokohama Kanagawa JAPAN
TEL +81-45-717-7026 / FAX +81-45-717-7027 / e-mail: info@badland.net



Comment On Facebook